In response to a series of superbug outbreaks around the country, some doctors and hospitals are trying out disposable scopes to combat the spread of antibiotic-resistant bacteria.
U.S. regulators recently approved two new colonoscopes designed to be used just once and thrown away. They will sell for $250 or less apiece — compared to roughly $40,000 or more for a conventional scope that lasts several years but must be disinfected after each use. Other companies are promoting such devices — flexible, lighted tubes used to peer deep inside the body — for use in the lungs and kidneys.
The new scopes are coming primarily from smaller companies looking to challenge a handful of dominant device makers. The upstarts are seizing on growing evidence that many reusable instruments cannot be cleaned reliably, even when manufacturer’s instructions are followed.
“If you can tell patients we have a disposable device so there’s really no chance of infection, that has to be very appealing,” said Chris Lavanchy, engineering director at the ECRI Institute, a nonprofit organization that tests medical devices. “This could allay public fears.”
Scopes include a wide array of devices used on millions of patients annually. As they snake through a patient’s throat, intestines and other cavities, they pick up mucus, blood and thousands of microbes. But the delicate devices can’t be sterilized like a scalpel because intense heat would destroy crucial components.
Instead, the scopes are brushed and washed with disinfectants in preparation for the next patient. Despite those efforts, contamination can persist and drug-resistant bacteria can result in patient infections that are difficult or even impossible to treat.
The threat has led to safety alerts from the Food and Drug Administration and a recent U.S. Senate investigation into repeated failures by manufacturers and hospitals to report outbreaks.
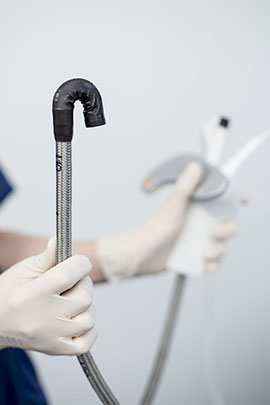
A disposable colonoscope from German device maker Invendo Medical secured clearance from the FDA in August. (Courtesy of Invendo)
Overall, as many as 350 patients at 41 medical facilities worldwide were exposed to or infected by contaminated gastrointestinal scopes from 2010 to 2015, according to the FDA. And at least 35 patients at U.S. hospitals have died since 2013 after developing infections tied to tainted scopes, according to hospitals and public health officials.
For now, just a handful of medical centers are experimenting with the disposable colonoscopes. Some doctors remain skeptical about whether a cheaper scope will provide the high-quality images and versatility they need to diagnose and treat patients effectively. Many doctors count on the sophisticated cameras on existing scopes as well as multiple channels inside the device to accommodate surgical instruments.
Dr. Simon Lo, a nationally known gastroenterologist at Cedars-Sinai Medical Center in Los Angeles, said he shares those concerns. Nonetheless, he said he’s eager to try the disposable colonoscope from German device maker Invendo Medical in the coming months. The scope secured clearance from the U.S. Food and Drug Administration in August.
“I’m not totally sold this will be comparable to the [conventional] scopes other companies have spent decades perfecting. It’s almost too good to be true with it being so cheap,” Lo said. “But this is a fantastic possibility and at least gives us an alternative to the current scopes.”
Physician groups such as the American Society for Gastrointestinal Endoscopy maintain that the overall risk of infection is very low from these reusable devices. They say the benefits of screenings and many other procedures far outweigh any potential danger.
But some doctors say a simpler, single-use scope could be sufficient for many routine examinations. It could also be preferred for immunosuppressed patients who are more susceptible to infection and for patients who have already tested positive for antibiotic-resistant bacteria and would be likely to spread it.
“For those patients, it would be great to use this and throw it away,” Lo said.
His hospital, Cedars-Sinai, reported four infections last year from contaminated duodenoscopes, which are inserted down a patient’s throat and used to treat problems in the digestive tract such as cancers and blockages in the bile duct.
Colonoscopes, which look at the inner lining of the large intestine, haven’t been linked to the recent outbreaks, but concerns about cleaning and the spread of bacteria apply to all types of reusable scopes. Last year, the FDA warned about the risk to patients posed by bronchoscopes, used to examine problems in the airway and lungs.
In one study, researchers found that more than 75 percent of colonoscopes and gastroscopes were still contaminated after cleaning and disinfection in accordance with manufacturer guidelines.
Hospitals have experimented with a wide variety of new safety measures over the past two years. Some facilities started testing scopes for contamination after cleaning and holding them in quarantine for 48 hours to check them again for bacterial growth.
Those steps added layers to what was already a labor-intensive process. It costs about $75 or more to clean a scope each time.
“There is a lot of time and money tied up in that,” said John Cifarelli, chief commercial officer for Invendo. “The best solution is a device that doesn’t have to be cleaned.”
The leading scope makers haven’t shown much interest thus far in single-use devices, which could affect their longstanding dominance in the business.
Olympus Corp., which controls 85 percent of the U.S. market for gastrointestinal scopes, didn’t respond to a request for comment. The Tokyo-based company, linked to numerous infections in the U.S. and Europe, conducted a voluntary recall of its duodenoscopes this year and made repairs designed to reduce the contamination risk.
In a statement, another big manufacturer, Fujifilm, said it “has no plans at this time to market single-use disposable scopes, and cannot speak to the benefits or risks associated with such products.”
Israeli company GI-View received FDA clearance in August for its single-use colonoscope, called Aer-O-Scope, priced at about $200. The company’s chief executive, Tal Simchony, acknowledges that his smaller company faces an uphill battle against the industry giants. But he said he’s optimistic it can address the “ick” factor some patients feel with a reusable scope.
Other companies see opportunity, too. Boston Scientific, a bigger device maker, promotes a disposable ureteroscope to aid in treatment of kidney stones and other procedures. Ambu A/S, based in Denmark, has sold a single-use bronchoscope for about $300 in the U.S. for the past few years.
In Europe, two biomedical engineers are raising money to build a prototype and manufacture what could become a full line of disposable endoscopes. Francisco Soriano, one of the engineers in Barcelona, said he learned the business from repairing scopes with his father, who was a longtime Olympus technician.
“We must reinvent the endoscope as we know it,” Soriano said. “Our vision is to eliminate cross-infection completely.”