Jason was hallucinating. He was withdrawing from drugs at an addiction treatment center near Indianapolis, and he had hardly slept for several days.
“He was reaching for things, and he was talking to Bill Gates and he was talking to somebody else I’m just certain he hasn’t met,” his mother, Cheryl, says. She remembers finding Jason lying on the floor of the treatment center in late 2016. “I would just bring him blankets because they didn’t have beds or anything.”
Cheryl had taken Jason to the clinic out of desperation. Jason, now in his late 30s, has struggled with addiction since he was a teenager. Cheryl saw his drug use escalate after he was prescribed a benzodiazepine for his anxiety, and he eventually began using heroin and meth. Over the years, Jason would try to get into recovery, but treatment programs didn’t help him for very long.
“I thought he was going to die,” Cheryl says. (KHN and NPR are using only first names because Jason worried he would lose his job if his employer found out about his addiction history.)
In late 2016, she saw a local TV news segment about a clinic called Emerald Neuro-Recover. The staff there treats addiction with something called NAD therapy, an IV infusion that can contain amino acids and other nutritional supplements, including nicotinamide adenine dinucleotide, a compound found in living cells.
The infusion, which is delivered over 10 to 15 days, cost $15,000, and it wasn’t covered by insurance. But the TV report said Emerald’s treatment was “proven to wipe drug cravings away.” Cheryl was intrigued.
Emerald and dozens of other companies across the U.S. say NAD therapy can address conditions from anxiety to depression to chronic fatigue and even Alzheimer’s.
And clinicians offering the treatment say that it reduces or stops cravings for alcohol or illicit drugs in up to 90% of patients. The treatment has gained attention on addiction recovery blogs and in the mainstream media.
But such claims about NAD therapy and addiction are not supported by scientific evidence, and they may conflict with federal and state regulations against deceptive marketing of medical treatments. Emerald and other addiction treatment clinics use these claims on websites, social media and in the news to attract clients looking for help. Emerald even used patients’ stories to promote the therapy — in some cases, more than a year after the patients returned to using illicit drugs.
In an interview with Side Effects Public Media, Emerald leadership defended its use of the therapy. “It’s not really controversial; it’s just novel or new,” says John Humiston, a family medicine physician and the company’s medical director. “The cravings we expect to be gone within days.”
Earlier this year, Emerald leadership discussed NAD therapy with Side Effects but cut the interview short amid questions about the treatment’s efficacy. Company officials declined another interview and did not respond to follow-up questions via email. For that reason, Side Effects was unable to ask them about Jason’s case.
Treatment centers touting high success rates can sound appealing to vulnerable people suffering from addiction or to their families, even if there’s no solid evidence to support their methods. “[Clinics] know this is a really desperate population,” says Basia Andraka-Christou, a health policy researcher focused on substance use disorders at the University of Central Florida.
Unsubstantiated claims have long been a part of addiction treatment. For instance, in the late 19th century, a doctor dubbed his formula the “Double Chloride of Gold Cure” and sold it via mail order for addiction, claiming a 95% cure rate. “In a week the desire to drink will be gone,” read one advertisement.
More recently, NAD therapy is among a wide range of unproven treatments currently marketed to people with addiction, including the herbal extract kratom and other types of supplements. The FDA and the FTC cracked down on a few of these last year but have limited resources to police the market for unproven treatments. And that leaves consumers on their own to sort out fact from fiction.
While patients spend time and money on ineffective treatments, they miss out on proven therapies that can reduce their risk of relapsing, including behavioral counseling and medications approved by the FDA for treating addiction, says Andraka-Christou. “We do actually now have evidence-based treatments available,” she says. “But you still do have these quack treatments popping up.”
A vial containing the mix of supplements that Emerald uses in its infusion treatments.(Jake Harper/Side Effects Public Media)
A Hard Sell
Numerous companies make bold claims about NAD therapy. A Las Vegas clinic says, “IV NAD+ therapy has a 90% success rate at reducing cravings and a 7% relapse rate.”
A clinic in Pooler, Ga., says NAD therapy can provide “rapid reduction or even elimination of cravings, restoring clarity of mind and enthusiasm to be alive.”
Another center in Greenville, S.C., says, “Withdrawal signs of addiction go down approximately 70-80% on the first day and continue to decline as the therapy progresses.”
Similar glowing testimonials from Emerald led Cheryl and Jason to meet with Emerald leadership in late 2016, including founder Joe Pappas and patient liaison Amora Scott. Cheryl recalls, “They said, ‘This is going to fix it. … It has never not worked for us. It works for everyone.'”
Jason insisted his mother shouldn’t pay thousands of dollars for his treatment. She had already spent too much money on him. They decided not to come back.
“Well, then Amora started calling me and calling me and calling me,” Cheryl says. Unknown to Jason at the time, Cheryl says Scott persuaded her to pay for the treatment upfront.
Cheryl took out an advance on her credit card and met Scott at a gas station to hand over the money. “When I gave her that check, I looked at her and said, ‘This is to save my son’s life,’ ” Cheryl recalls.
Fifteen thousand dollars could seem like a bargain for such a quick fix — one that “restore[s] the brain to its pre-addiction neurologic state,” according to a press release from Emerald.
But there has been little research on the effects of the formulas used by Emerald and similar clinics.
“I don’t know where those claims could come from, but it doesn’t seem realistic to me,” says Emily Zarse, an addiction psychiatrist in Indianapolis. She says there’s insufficient evidence to support using NAD therapy over other standard treatments: “There’s no actual data on any of these things.”
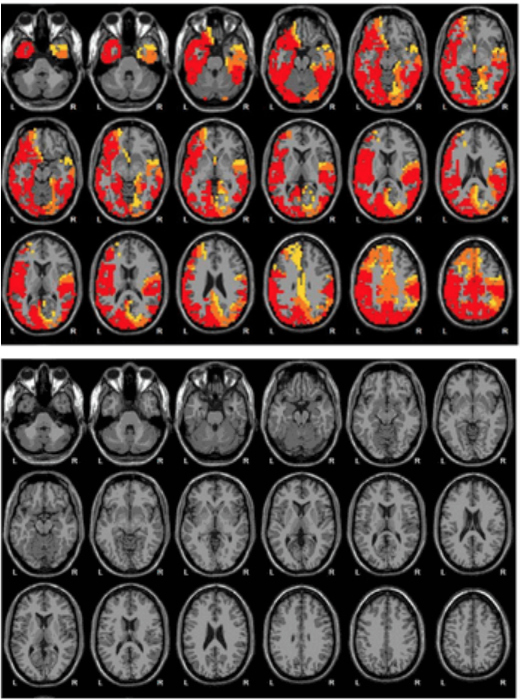
Brain scans from Emerald show what it claims to be before (top) and after (bottom) images of a woman’s brain following NAD treatment. The images appear in an Emerald brochure, including text that says, “The brain is more calm after 12 days of NAD+ Amino Acids Therapy.”(Emerald/Screenshot by Side Effects Public Media)
For an additional $400 fee, Emerald patients can have their brain scanned at a nearby clinic to document their progress with NAD therapy. An Emerald brochure shows a series of scans from a woman whose “brain is suffering from alcoholism.” Areas that glow red, orange and yellow — “HYPERACTIVE and OVERACTIVE” — totally disappear from the scans after 12 days of NAD therapy, according to the company.
“This is totally bogus,” says Leslie Hulvershorn, an addiction psychiatrist at the Indiana University School of Medicine with expertise in brain imaging who reviewed the images via email. “We do not have research in our field that allows us to use EEG or any other brain imaging technique to document treatment response.”
NAD, which is an important coenzyme in several cellular processes, including energy metabolism, is being researched at Harvard for its role in aging. Supplements claiming to boost NAD levels have recently gained popularity for purported anti-aging benefits. But NAD’s benefits in addiction treatment are unproven, and providers cite unpublished research to make sweeping claims.
One pilot study cited among some NAD therapy providers shows close to 90% of patients have reduced cravings after 10 days of treatment. The study falls short of the standard used by the scientific community to weigh evidence: It did not compare NAD therapy to a placebo or other treatment. It also did not undergo rigorous peer review, and the results have not been published in a scientific journal.
A doctor involved with that study, Richard Mestayer, says he is used to skepticism. Mestayer runs a clinic in Springfield, La., that offers NAD therapy. He says it is unclear how NAD therapy helps with addiction but that his personal experience convinced him it works.
“I think there’s a lot of stuff we don’t know yet,” he says. “I was a skeptic, but when a two-by-four hits you in the head every time, you say, ‘Oh, I better pay attention.'”
Dangerous Withdrawals
The hallucinations started several days into Jason’s treatment at Emerald. Cheryl wanted to take him to the emergency room.
Rapidly withdrawing patients from benzodiazepines can cause dangerous side effects, such as seizures — it can even be fatal, says Zarse. “There are two types of withdrawal symptoms that can kill you: alcohol and benzodiazepines,” she says. “It can cause enough misfiring in the brain that it can lead to brain death.”
The standard treatment is to slowly wean someone off benzodiazepines. “They even give benzos for benzodiazepine withdrawal in jail — that shows you how serious this is,” Zarse says.
Still, Cheryl says, Emerald staff told her to take Jason home rather than to the hospital. She decided to go to the ER anyway after Jason tried to throw himself through a wall.
Jason was still hallucinating when he arrived at the ER, and then the seizures started. “He was just totally out of it for about three days,” Cheryl says. “Not even alert.”
One of the doctors who treated Jason noted in his medical records: “Unclear exactly what this NAD substance/medication is.”
When Jason left the hospital, he returned to Emerald to finish the treatment. “I didn’t know what else to do,” he says.
Jason says the therapy didn’t work. He white-knuckled his way through abstinence for three months before he relapsed. “One day out of the blue, I called somebody up and just was going to do it one time,” he says. “You know how that goes.”
Marketing Unapproved Substances
The federal Food and Drug Administration has not approved NAD therapy, according to a spokesperson for the agency.
Substances marketed as treatments for specific conditions are considered medications and must be approved by the FDA for that purpose, says Andraka-Christou. For medications, FDA approval requires three phases of human clinical trials. Without that approval, it would violate FDA regulations to market a treatment for that condition.
More broadly, making unsupported claims about a medical treatment or supplement violates federal rules. Both the FDA and the Federal Trade Commission regulate how companies advertise treatments and supplements.
But no publicly available information could be found to show that either agency has taken enforcement action against any clinic offering NAD treatments.
Spokespeople for the FDA and the FTC said via emails that their agencies could not comment on specific cases. “All advertising under our jurisdiction must be true, not deceptive, and supported by competent and reliable scientific evidence,” wrote the FTC spokesperson.
“The FDA takes action against companies that engage in ‘health fraud,'” said the email from the FDA.
Lack of FDA action doesn’t mean it is acceptable for clinics to market the therapy, says Chris D’Adamo, an assistant professor at the University of Maryland who researches dietary supplements.
“The FDA can be slow, and it’s understandable because there are so many [potential enforcement issues] out there,” he says. “There could still be cause for concern.”
Patient Stories
Since its inception, Emerald has featured patients’ stories on social media and in news coverage, much of which uncritically repeats the company’s claims about ending addiction. But several of these same patients went to jail for drug and alcohol offenses soon after being treated at Emerald.
In a 2017 TV news story about Emerald, a man says that Emerald helped him get his alcohol and pill addiction under control. Reached by phone, he told Side Effects that he reluctantly said those things to get the TV interview over with. “[NAD therapy] was a complete waste of my time and my family’s money,” he said. “It did absolutely nothing for me.” (He asked to remain anonymous because many of his family and friends don’t know about his addiction, and he worries about his future job prospects.)
He added that he also experienced a seizure when the doctor quickly cut him off from alcohol without antiseizure medication. He says he started drinking again about a week after he finished NAD therapy, and he was arrested for drunken driving a few months later.
In another video Emerald posted on YouTube in 2017, an Indianapolis man is seen leaving Emerald on a sunny day. “I feel wonderful,” he says. “Using heroin, I had a lot of racing thoughts, anxieties, cravings. All that’s gone.” He tells other people who use heroin to go to Emerald.
Six months later, he was in jail for possession of a syringe. Reached by phone, he said that the treatment didn’t work for him, and that he received it free of charge.
Emerald still promoted patients’ stories like these on social media until December 2018. The company began removing content from its website, YouTube and Facebook shortly after Side Effects began reporting this story.
Emerald executives declined to provide Side Effects with a patient to interview.
Asked about cases of relapse among Emerald patients, Humiston replied: “What I’ve seen is that [the treatment is] very effective.” Humiston started work at Emerald in January 2019, but he was a medical adviser for the company before then, and emails between Cheryl and Emerald staff indicate that he was consulted about Jason’s treatment there.

Dr. John Humiston and Star Voigt, former CEO of Emerald, in January at the Emerald clinic.(Jake Harper/Side Effects Public Media)
Origins Of Treatment
Humiston says he believes in the treatment he offers: “It’s got quite a reputation of success. Nothing’s 100%, although for most people, it is 100%. That’s been my experience.”
But Humiston acknowledges that he does not regularly track patients’ long-term outcomes: “That’s the reason to get a study organized,” he says. Last year, Humiston told a local TV station that a clinical trial was forthcoming, but it has not materialized.
Humiston first learned about NAD therapy from a man named William Hitt. Hitt is often credited with originating the treatment, but he was not a doctor or a researcher. According to a lawsuit brought by the state of Texas in the mid-’80s, he falsely claimed to be a doctor when he treated AIDS patients with “injections of the patient’s own filtered urine.” Forced to shut down in Texas, he moved to Tijuana, Mexico, where Humiston worked with him from 2003 until his death in 2010.
Humiston himself has had trouble with his medical license. The Medical Board of California reprimanded him, according to investigation documents, for committing “gross negligence in his care and treatment” of his teenage son, who almost died in 2016 when Humiston failed to seek proper treatment for the boy’s heart infection. Documents say Humiston began performing IV treatments on the boy before he was 3 years old, which may have caused the boy’s heart issues.
Asked about the investigation, Humiston said there was “inaccurate information put in there” but that he accepted a public reprimand from the medical board “just to end it.” He did not respond to emailed follow-up questions about the disciplinary case.
Humiston applied for an Indiana medical license in November 2018, and the state granted it. He became Emerald’s medical director in January. He is at least the sixth doctor to work with the company in its three years of operation.
‘I Owe Her The Money’
When asked in January about Emerald’s claims and the origins of NAD therapy, Star Voigt, the CEO at the time, declined to answer further questions. “We’re trying to help people,” she said. “So if you’re going to go into that, then I’m going to ask you kindly to leave.”
Side Effects sent further questions via email, but the company did not answer them. Instead, Voigt sent a statement from Humiston expressing concern that Side Effects’ reporting wouldn’t be balanced or objective. Voigt left the company soon after.
Cheryl, the patient Jason’s mother, wrote to Emerald founder Pappas a few months after her son left Emerald. She told him that Jason was facing an $11,000 medical bill from his hospital stay and that he still struggled to stay away from illicit drugs. She reminded Pappas that stopping benzodiazepines cold turkey — what Jason went through at Emerald — is dangerous and goes against standard medical practice.
Cheryl wanted a refund so she could pay off Jason’s medical bill. “Can we compromise?” she wrote.
Scott, the patient liaison, wrote back that Humiston believed Jason should be tested “for mold … infections, and/or inflammation in the blood and body.” Instead of a refund, Emerald offered further NAD treatments and another therapy — for $3,000.
Cheryl and Jason declined the offer. “First, do no harm,” Cheryl wrote back. She filed complaints with the FTC and the state attorney general, but nothing came of it. (Indiana law allows the state attorney general to prosecute companies for deceptive advertising. The office would not confirm or deny whether it is investigating Emerald’s practices.)
The hospital eventually did waive the $11,000 bill. But Cheryl still has not received a refund from Emerald.
“I feel like I owe her the money,” Jason says. “At some point, I’ll pay it back.” He says he finally got help with his addiction through a local 12-step program that he has been part of for two years. Looking back at his treatment at Emerald, he says he felt duped into trying NAD therapy. “I think it’s taking advantage of people.”
“I can’t believe that no one stops them,” Cheryl says. “You’ve got these people selling snake oil, and they’re getting away with it.”
This story is part of a partnership that includes Side Effects Public Media, NPR and Kaiser Health News.







