FDA Announces Recall of Heart Pumps Linked to Deaths and Injuries
Some pumps used in end-stage heart failure caused a buildup of biological material that blocks blood flow from the device to the heart’s aorta. The FDA’s recall affects nearly 14,000 devices.
Swap Funds or Add Services? Use of Opioid Settlement Cash Sparks Strong Disagreements
The national opioid settlements don’t prohibit using money for initiatives already supported by other means, but doing so could dilute the impact.
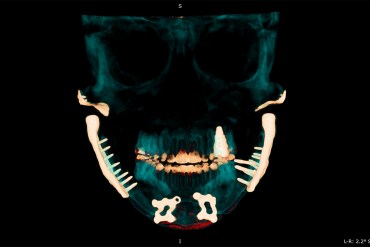
The Horrors of TMJ: Chronic Pain, Metal Jaws, and Futile Treatments
TMJ disorders affect as many as 1 in 10 Americans and yet remain poorly understood and ineffectively treated. Many common treatments used by dentists lack scientific evidence.
Track Opioid Settlement Payouts — To the Cent — In Your Community
Want to know how much opioid settlement money your city, county, or state has received so far? Or how much it’s expecting in the future? Use our new searchable database to find out.
A Mom’s $97,000 Question: How Was Her Baby’s Air-Ambulance Ride Not Medically Necessary?
There are legal safeguards to protect patients from big bills like out-of-network air-ambulance rides. But insurers may not pay if they decide the ride wasn’t medically necessary.
Los hirieron en el desfile del Super Bowl: un mes después se sienten olvidados
Durante el primer mes, los líderes comunitarios de Kansas City han discutido cómo atender a las personas que quedaron atrapadas bajo el fuego cruzado y cómo distribuir los más de $2 millones donados a los fondos públicos para las víctimas bajo el doloroso impacto inicial.
Social Security Chief Testifies in Senate About Plans to Stop ‘Clawback Cruelty’
Commissioner Martin O’Malley testifies to two Senate panels that his agency will stop the “injustices” of suspending people’s monthly benefits to recover alleged overpayments. The burden will be on the Social Security Administration to prove the beneficiary was to blame.
They Were Injured at the Super Bowl Parade. A Month Later, They Feel Forgotten.
In the first of our series “The Injured,” a Kansas family remembers Valentine’s Day as the beginning of panic attacks, life-altering trauma, and waking to nightmares of gunfire. Thrown into the spotlight by the shootings, they wonder how they will recover.
Exclusive: Social Security Chief Vows to Fix ‘Cruel-Hearted’ Overpayment Clawbacks
New Social Security Commissioner Martin O’Malley is promising to change how the agency reclaims billions of dollars it wrongly pays to beneficiaries, saying the existing process is “cruel-hearted and mindless.”
New York Joins Local Governments in Erasing Billions in Medical Debt
New York City is the latest jurisdiction to buy and forgive a backlog of unpaid medical bills for its residents. Local governments across the country, including in the Chicago area, are doing the same to reduce debt burdens for lower-income residents.
In This Oklahoma Town, Most Everyone Knows Someone Who’s Been Sued by the Hospital
Hospitals nationwide face growing scrutiny over how they secure payment from patients, but at one community hospital, the debt collection machine has been quietly humming along for decades.
In Year 6, KFF Health News-NPR’s ‘Bill of the Month’ Helps Patients in a Changing System
In the sixth year of the KFF Health News-NPR “Bill of the Month” series, patients shared more than 750 tales of medical billing problems, and reporters analyzed more than $730,000 in charges — including more than $215,000 owed by 12 patients and their families.
‘AGGA’ Inventor Testifies His Dental Device Was Not Meant for TMJ or Sleep Apnea
The FDA and Department of Justice are investigating the Anterior Growth Guidance Appliance, or “AGGA.” TMJ and sleep apnea patients have filed lawsuits alleging the device harmed them. Its inventor now says the AGGA was never meant for these ailments.
The Year in Opioid Settlements: 5 Things You Need to Know
In the past year, opioid settlement money has gone from an emerging funding stream for which people had lofty but uncertain aspirations to a coveted pot of billions being invested in remediation efforts. Here are some important and evolving factors to watch going forward.
Deep Flaws in FDA Oversight of Medical Devices, and Patient Harm, Exposed in Lawsuits and Records
Thousands of medical devices are sold, and even implanted, with no safety tests.
‘I Am Just Waiting to Die’: Social Security Clawbacks Drive Some Into Homelessness
The Social Security Administration is reclaiming billions of dollars in alleged overpayments from some of the nation’s poorest and most vulnerable, leaving some people homeless or struggling to stay in housing, beneficiaries and advocates say.
When a Quick Telehealth Visit Yields Multiple Surprises Beyond a Big Bill
For the patient, it was a quick and inexpensive virtual appointment. Why it cost 10 times what she expected became a mystery.
Patients Facing Death Are Opting for a Lifesaving Heart Device — But at What Risk?
The HeartMate 3 is considered the safest mechanical heart pump of its kind, but a federal database contains more than 4,500 reports in which the medical device may have caused or contributed to a patient’s death.
New Doula Benefit ‘Life-Changing’ for California Mom
Doulas, independent workers who act as advocates for birthing parents, have been shown to help prevent pregnancy complications and improve the health of both mothers and babies. California’s Medicaid program started covering their services this year, but some doulas say bureaucratic obstacles and inadequate pay prevent their effective use.
Social Security Chief Apologizes to Congress for Misleading Testimony on Overpayments
Acting Commissioner Kilolo Kijakazi sent the letter days after KFF Health News and Cox Media Group reported that the agency has been demanding money back from more than twice as many people as she’d disclosed in October testimony.